I-MASK+ Prevention & Early Outpatient Treatment Protocol for COVID-19


Below you can download the I-MASK+ Prevention & Early Outpatient Treatment Protocol for COVID-19 with guidance on the timing and doses of each component medication. Further below please find more information on the I-MASK+ Protocol.
The I-MASK+ Protocol complements our MATH+ Hospital Treatment Protocol for Covid-19 from March 2020, which is intended for hospitalized patients. Both are physiologic-based combination treatment regimens developed by leaders in critical care medicine. All component medicines are FDA-approved, inexpensive, readily available and have been used for decades with well-established safety profiles. In October 2020, we added ivermectin as a core medication in the prevention and treatment of COVID-19.
The protocol document is available in several languages (see below) – more translations are available here. This is not a medical advice, but a recommendation – please consult your doctor, share the information on this website with her/him, and listen. Please review our Disclaimers!
Please check this page regularly for updates – new medications may be added and/or dose changes to existing medications may be made as further scientific studies emerge.SUBSCRIBE TO RECEIVE UPDATES
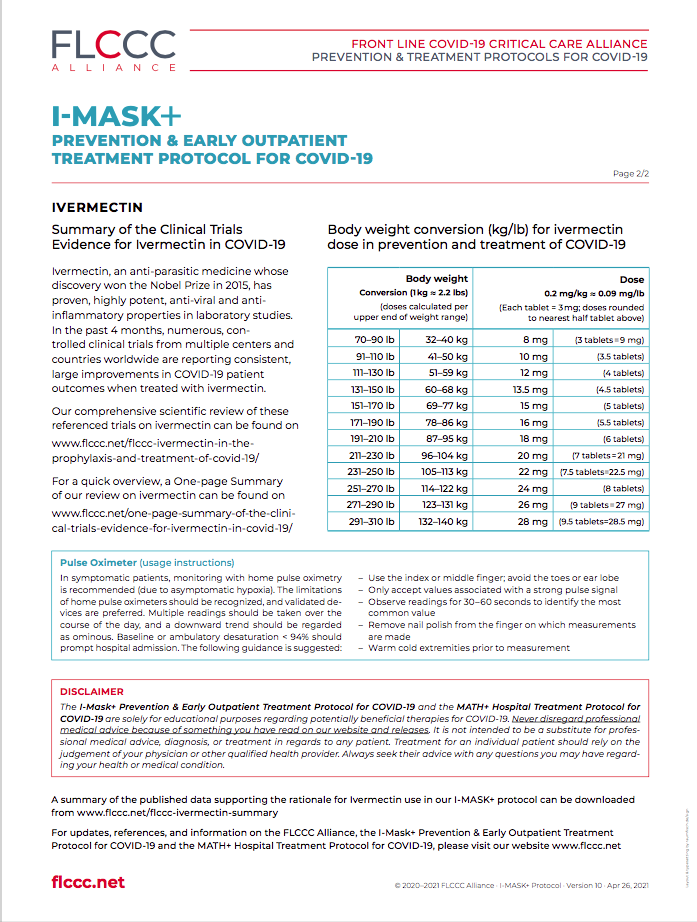
Current I-MASK+ protocol: version 10, updated on April 26, 2021.
Prevention & early outpatient treatment for COVID-19
About the I-MASK+ Protocol for COVID-19
In October 2020, the FLCCC Alliance developed a preventive and early outpatient combination treatment protocol for COVID-19 called I-MASK+. It’s centered around ivermectin, a well-known, FDA-approved anti-parasite drug that has been used successfully for more than four decades to treat onchocerciasis “river blindness” and other parasitic diseases. It is one of the safest drugs known. It is on the WHO’s list of essential medicines, has been given 3.7 billion times around the globe, and has won the Nobel prize for its global and historic impacts in eradicating endemic parasitic infections in many parts of the world. Our medical discovery of a rapidly growing published medical evidence base, demonstrating ivermectin’s unique and highly potent ability to inhibit SARS-CoV-2 replication and to suppress inflammation, prompted our team to use ivermectin for prevention and treatment in all stages of COVID-19. Ivermectin is not yet FDA-approved for the treatment of COVID-19, but on Jan 14, 2021, the NIH changed their recommendation for the use of ivermectin in COVID-19 from “against” to “neutral”. (see our press release).
Our life-saving MATH+ Hospital Treatment Protocol for COVID-19 (available in several languages), created in March 2020, is intended for hospitalized patients. The recently developed I-MASK+ Prevention & Early Outpatient Treatment Protocol for COVID-19 (this page) is designed for use as a prevention and in early outpatient treatment, for those who test positive for COVID-19. The protocols complement each other, and both are physiologic-based combination treatment regimens developed by leaders in critical care medicine. All the component medicines are FDA-approved (except ivermectin), inexpensive, readily available and have been used for decades with well-established safety profiles.
Please download and share our I-MASK+ Prevention & Early Outpatient Treatment Protocol for COVID-19. (It is currently being translated into several languages).
Below are a list of links to our one-page summary of the latest evidence for the protocol, plus videos of FLCCC Alliance doctors discussing the emerging evidence for the use of ivermectin in the prevention and treatment of COVID-19, and a short list of up-to-date studies and clinical trials on this topic.
I-MASK+ Prevention & Early Outpatient Treatment Protocol for COVID-19